In this post, we dive in to the similarities, differences, and advantages of corrective and preventive action so that you can improved determine when to apply them within your Business.
Assure actions taken from the web-sites in reaction to the issue are coordinated to make sure the difficulty is systematically tackled.
An overview of the CAPA approach and how it can help with recurring issues and stop unwelcome scenarios.
MasterControl CAPA software digitizes and automates CAPA procedures including routing, notification, escalation and approvals to prevent bottlenecks and expedite difficulty resolution.
The justification for extension shall be prepared by the worried Section and sent for checking to the concern department head and QA Section/internet site quality head.
What is the complete type of : Expense for every Mile means Price tag for each Mile. It can be also known as Expense for each thousand because the term "mille" in Latin usually means "1 thousand." It is just a internet marketing time period that refers to the price of 1,000 ad impressions on...
The CAPA procedure would be the initial doc that the inspectors and auditors assessment prior to the complete CAPA sub-method.
Corrective Action refers to the whole process of reacting to the issues/defects on the item, purchaser complaints or nonconformity and resolving them.
A proactive system known as preventive action is used to place attainable challenges or nonconformities right before they occur also to get ways to stop them from going on. Corporations use it as a vital ingredient of top quality administration systems to regularly improve their functions, goods, and products and services.
A preventive action, However, is a lengthy-term Resolution to reduce the likelihood of issues that the team may possibly come upon.
The corrective action takes place when the defect is detected. In contrast, preventive action more info takes place right before detecting the defect.
Corrective and preventive action (CAPA or just corrective action) is made of enhancements to a company's processes taken to do away with triggers of non-conformities or other unwanted cases. It will likely be a set of actions, rules or laws demanded by a company to take in production, documentation, procedures, or systems to rectify and eradicate recurring non-conformance. Non-conformance is determined immediately after systematic analysis and Investigation of the root cause of the non-conformance.
The standard department is responsible for getting ready, examining, and approving the CAPA forms through the entire organization.
A five whys template is accustomed to resolve the root reason for a problem making sure that click here business groups can keep away from recurrence. This may be utilized by top quality Management teams to assist CAPA Reporting.
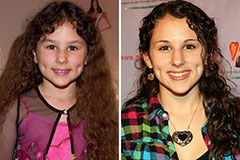 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Brandy Then & Now!
Brandy Then & Now! Melissa Joan Hart Then & Now!
Melissa Joan Hart Then & Now! Barry Watson Then & Now!
Barry Watson Then & Now! Richard Thomas Then & Now!
Richard Thomas Then & Now!